
Giving Huntington's disease the finger? Two teams report success for zinc-finger drugs in cells and mice
Success in Huntington’s disease cells and mice for zinc finger drugs that reduce production of the harmful protein

Designing drugs that tell our cells to make less of the harmful mutant huntingtin protein is one of the most promising approaches to treating Huntington’s disease. Most huntingtin-lowering attempts so far have tried to ‘shoot the messenger’ rather than attacking the source of the message – the DNA itself. Now, two independent reports of success in HD mice have given a boost to ‘zinc finger’ drugs – which interact directly with the HD gene itself. It’s early days for this new technology: what do we know, and what challenges are ahead?
What on earth are zinc fingers?
Zinc is a shiny metal found in ‘silver’ coins, batteries and white paint. But our own bodies use zinc for an intriguing purpose – which researchers hope to hijack, to fight Huntington’s disease. It turns out that zinc is vitally important for enabling cells to control the activity levels of different genes in our DNA.
Remember that a gene is a set of instructions, spelled using the chemical ‘letters’ A, C, G and T. Each gene has a different sequence of letters, and cells use zinc-containing proteins to help control individual genes based on their specific sequence of letters.
When zinc hooks up with a gene-controlling protein, it forms ‘zinc finger proteins’ – so called because they can poke into the tight groove between the two strands of DNA and touch the sequence they’re made for.
A single zinc finger can tickle any three letters, depending on the arrangement of the zinc and protein bits that make it up. So one zinc finger could stick to the sequence ‘ATG’, while another might go for ‘CAG’.
The exciting bit is that individual zinc fingers can be joined together in sequence. Joining those two together, you’d end up with one molecule that sticks to the 6-letter sequence ATG-CAG.
Designer fingers
After decades of work decoding and understanding the workings of DNA and our genes, scientists can now create synthetic zinc fingers, engineered to stick to any DNA sequence they like.
What’s more, these synthetic zinc finger molecules can be tweaked and packaged with other drugs, to produce multi-purpose DNA-binding tools that can stick, slice, glue and block.
Because Huntington’s disease is caused by a single faulty gene, it’s a good candidate for zinc finger drug research.
Huntington’s disease happens when a person has a spelling mistake in the gene that tells cells how to make a particular protein – called huntingtin. When a person has too many CAGs in a row near the start of the huntingtin gene, a harmful ‘mutant’ form of the protein is produced based on the instructions in the gene.
Blocking the HD gene at its source
Two groups of researchers- one an academic team from Barcelona, Spain; the other a biotech company from California called Sangamo – have just announced successful experiments using zinc finger drugs targeting the Huntington’s disease gene.
The Spanish team’s results were recently published in the journal PNAS. Sangamo’s findings were presented at the recent Society for Neuroscience meeting in New Orleans, where HDBuzz was in the audience.
Both teams designed zinc finger molecules that would stick to the ‘CAG tract’ of the huntingtin gene, and tell cells not to read the gene.
The zinc finger chains were chosen and honed to try to make them stick as much as possible to harmfully-long CAG stretches without sticking to normal-length stretches. The Spanish team catchily called this a ‘molecular tape measure’ approach.
After a bit of chemical tinkering, each team then tested their best candidate zinc finger recipes in cells growing in a petri dish.
“Once a zinc finger has targeted a particular DNA sequence, lots of exciting things are theoretically possible.”
Going viral
Unfortunately, scientists can’t just design and make a zinc finger drug and squirt it onto cells, or stick it in a pill. Being proteins, zinc finger drugs are big, complex and fragile. If swallowed as a tablet, the drug would be broken down by the digestive system.
Even if given as an injection into the blood, the zinc finger protein would not reach the brain, let alone get as far as the nucleus of our neurons, where it needs to be in order to do its work.
To solve this problem, researchers can use viruses to ‘hitch-hike’ into the nucleus of cells.
Having chosen the zinc finger protein chain they want, it’s fairly straightforward to design a DNA sequence that will tell cells to make that very protein. That DNA sequence can then be stuck into particles of a virus called AAV, which is harmless, but good at infecting neurons.
When the virus meets a suitable cell, it injects its DNA cargo into the cell, turning the cell into a factory for making the zinc-finger drug!
Testing in cells
The Spanish team honed their top zinc finger candidates in several types of different genetically-engineered cells and cells grown from the skin of Huntington’s disease patients. The Sangamo researchers used these HD patient cells, too, as well as brain cells from an HD mouse model.
Both teams’ zinc finger drugs did the job of reducing the activity of genes containing long CAG stretches.
Off-target effects
There’s a possible snag here. The huntingtin gene isn’t the only one with a long CAG stretch – lots of other genes do as well. So, a drug that targets CAG tracts could switch off those genes too, which could end up doing more harm than good.
A quirk of the huntingtin gene might be helpful when it comes to avoiding these ‘off-target effects’. The CAG tract in the huntingtin gene happens to be very close to the start of the gene, where the effects of zinc-finger drugs act most strongly.
Both groups took measurements to see whether other genes were being affected by the zinc-finger drug, and found results that were broadly reassuring. Any effects were small compared with the desirable action on the mutant huntingtin gene.
Bring on the mice
The Spanish team, who are a bit further on in their work, took things to the next level by testing their best zinc finger candidate in a Huntington’s disease mouse model.
They gave the mice a single injection into the brain, containing virus particles packed with DNA instructions making the zinc finger drug.
Activity of the mutant huntingtin gene was reduced by around 50% in the brain near the injection site. The appearance of blobs of mutant huntingtin protein was reduced by about 40%, too. There was no evidence of harmful effects like weight loss, but the study was too small to know whether the zinc finger drug improved symptoms.
Hold on a minute – isn’t this just gene silencing?
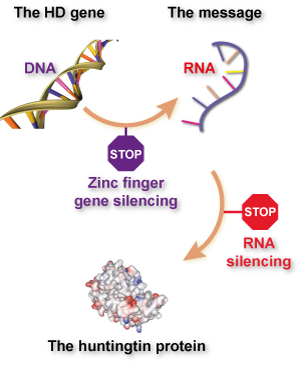
If what you’ve read here about zinc finger technology reminds you of ‘gene silencing’ or ‘huntingtin-lowering’ methods for treating Huntington’s disease, give yourself a gold star. What these groups are attempting basically is ‘gene silencing’, with a twist – it’s aimed directly at the DNA, rather than targeting a ‘message molecule’ called RNA.
So far, most attempts to lower huntingtin production have focused on the RNA message molecule, because it’s easier to design drugs that target RNA than DNA. RNA floats around in cells in a single strand, while DNA is hidden away in our cells’ nucleus. What’s more, RNA silencing drugs have been around longer, and some don’t need to be packaged up in virus particles.
Since RNA-based gene silencing drugs have been successful so far, why bother with the greater challenge of targeting the DNA of the huntingtin gene itself, especially if it means dealing with virus particles and big, fragile drugs made of protein? It’s a reasonable question, and there are three main answers.
The first is to do with getting to the root of Huntington’s disease. We know for sure that the mutation in the huntingtin gene is the ultimate reason why people get HD. Going after the DNA with zinc fingers means targeting the known cause of the disease. While RNA is an essential step in making the mutant protein, it’s one step removed from the ultimate cause. Zinc finger enthusiasts believe it’s worth attempting to overcome the additional hurdles of developing DNA-targeting drugs, because of the chance that the end result may be safer and more effective.
The second concerns RNA. Traditionally, biologists have assumed that RNA doesn’t directly do useful or harmful things – it simply sits there carrying information and being read by cellular machinery. However, we now know that there’s more to it than that, and there are several genetic diseases where the RNA is directly poisonous to cells, rather than simply carrying a harmful message.
While everyone agrees that the mutant huntingtin protein is the main cause of harm in Huntington’s disease, some researchers think that huntingtin RNA could be an additional source of damage. Others disagree, but if the RNA might be harmful, it does seem ideal to prevent it being made, rather than get rid of it afterwards.
The final reason is about going beyond silencing. There’s more to zinc fingers than reducing gene activity. Once a zinc finger has targeted a particular DNA sequence, lots of exciting things are theoretically possible.
Thinking many years in the future, it’s possible that zinc fingers could be used to direct molecular scissors and glue to the mutant HD gene, ‘snipping out’ the unwanted CAG repeats. That’s known as genome editing and it’s one of the approaches that Sangamo and others are interested in pursuing.
Upsides, downsides
Most of the HD researchers we speak to say that zinc fingers are “pretty cool” as a way of treating Huntington’s disease, and we’re inclined to agree. Fighting HD at the DNA level, the ultimate cause of the problem, is definitely something we should try, and we’re glad that progress has been reported independently by two groups after only a short time.
It’s important to bear in mind that it will take a long time to hone these techniques, which are still early on in the drug development pipeline – and things like genome editing will take decades to come to fruition for HD patients.
Meanwhile, everyone in HD remains excited about the ongoing efforts round the world to lower huntingtin levels by targeting the RNA. Those techniques are a lot further on, and should be entering human trials very soon.
Learn more
For more information about our disclosure policy see our FAQ…


