
Making babies: having a family, the HD way
Making babies: HDBuzz's feature article – updated for 2024 – on fertility technologies that can help at-risk people to have HD-free children
For people at risk of Huntington’s disease, having a baby who might inherit HD can make decisions around planning a family extremely difficult. This article explains the options available, and how modern reproductive science can make a difference right now to families touched by HD.
Content warning This article describes issues of fertility, tough choices, and medical procedures including termination of pregnancy.
Not all techniques described here are available everywhere, and in some countries, they can involve major expense. So, if you’re thinking about any of them, we recommend you contact a specialist genetic counsellor for individual advice. The earlier you do, the more options you’ll have.

Introduction
Many people with Huntington’s disease, or at risk of it, would like to know if there are ways to have children without passing the disease on to the next generation. The short answer is yes!
Genetic science and reproductive technologies mean that several choices are available to people who are at-risk of Huntington’s disease, to ensure that future children won’t be at risk of developing HD. This includes people who have had testing and carry a HD gene expansion, but there can also be options for some people who choose not to have a HD genetic test themselves.
First things first: nothing has to change
Although a lot of this article focuses on the options for having HD-free children, it is important to know that having a child without doing any genetic testing is very much an option for parents at risk of the condition.
As every HDBuzz article confirms, scientists are making real progress towards finding treatments for Huntington’s disease. While we cannot guarantee anything or promise a firm timeline, we firmly believe a time will come when at-risk children are born into a world where HD is a treatable condition.
Additionally, there is always a chance that the child won’t inherit HD gene expansion in the first place, and therefore will never develop HD.
Some people may want to guarantee HD-free children, but options may not be available to them, for example based on location, financial support, or religious beliefs.
Having a child at risk of HD is something that can be a point of discussion and debate within the HD community. While people may not agree with the decisions that others make, it is important to remember that everybody has the right to be respected when making their own decisions.
The Huntington Disease Youth Organisation has some resources available to help discuss HD and genetic risk to children in an age-appropriate way:
Some people feel that they don’t want to take any chances and would like to avoid the risk of passing on HD at all. That’s where genetic testing techniques come in. These options are available whether it is you or your partner who is at risk of HD.
What are my genetic testing options?
Thanks to genetic testing. we can identify the risk of HD for a fetus during a pregnancy, or in embryos in the lab.
Testing a fetus during pregnancy is called prenatal testing. Testing embryos in the lab is a form of in-vitro fertilisation or IVF, and is called pre-implantation genetic testing or PGT.
If you or your partner have had genetic testing that confirms you carry an HD gene expansion, you would be able to have direct testing during pregnancy or via PGT, to confirm whether or not the pregnancy or embryo has inherited the HD gene expansion.
Some people want HD-free kids without getting the gene test themselves. There are options for this, too! They involve more complicated versions of the same methods. So first we’ll discuss how it works for couples where one partner has already had a positive HD gene test.
Pre-implantation Genetic Testing (PGT)
Pre-implantation genetic testing is one way of having an HD-free kid without having to consider terminating a pregnancy. It can be a long, challenging and expensive process though.
PGT involves using eggs and sperm to create embryos in a lab, then performing the HD test on the embryos, and putting only the HD-negative embryos into the womb.
The PGT Process
PGT is IVF with an added step of genetic testing. IVF is a medical procedure which involves a hormone medication to cause the egg provider to produce more eggs than normal. Hormone medication can involve injections to deliver the medications into the body.
The eggs are then collected and fertilised using a sperm sample.
The fertilised eggs develop into embryos, which are grown in the laboratory for up to five days until they are a small bundle of cells. One or two cells are removed from each embryo at this stage and sent for genetic testing while the embryos are frozen and stored. Removing cells at this early stage of development doesn’t affect the way the embryo develops.
Any embryos found to be not at risk of developing HD will continue to be stored. Depending on the country you are in, one or two of these risk-free embryos are then transferred to the womb.
About two weeks after the embryos are transferred, a pregnancy test is run on a blood sample. If the transfer has been successful, pregnancy then carries on like normal.
The downside of PGT
The process of stimulating egg release, collecting eggs, fertilising them outside the body and returning embryos to the womb – is always a time-consuming and exhausting process. It can also be dangerous, carrying risks of the person becoming unwell.
Various things can go wrong, like not enough eggs or embryos being produced. There’s also more chance of having twins with PGT, which is harder work and riskier.
On top of the risks of the procedure, things can go wrong with the genetic bit of PGT. Embryos can be damaged when cells are removed, and sometimes the HD test doesn’t work because there isn’t enough DNA. Bad luck can mean that all the embryos have the HD mutation.
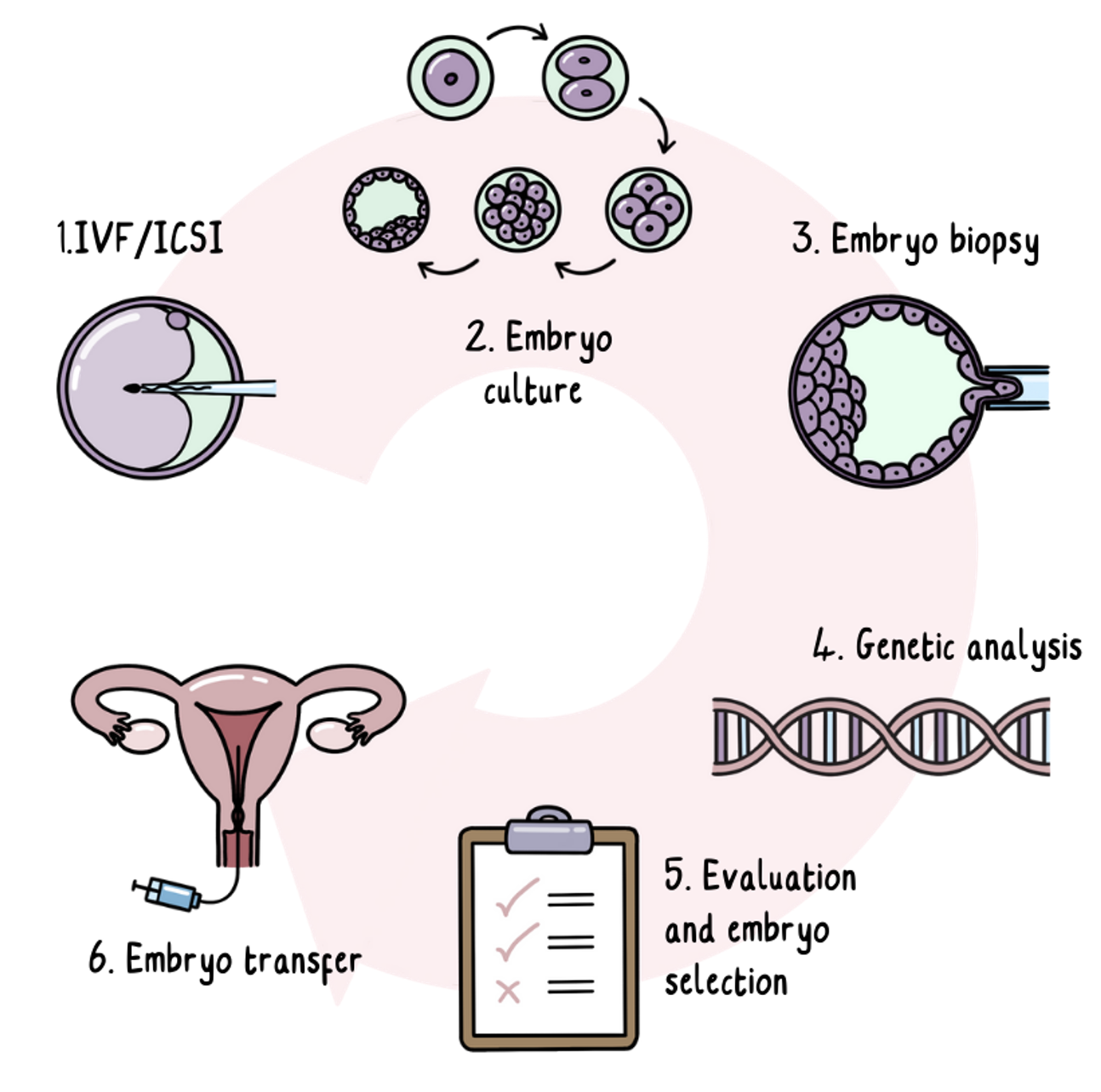
Image credit: @gcnotes
In the end, sometimes only one embryo is available for implantation – and sometimes none at all. To top it off, a pregnancy can fail after implantation. Overall, each attempt at PGT gives a 20-30% chance of an HD-free pregnancy. This success rate varies per PGT centre and is dependent on a number of factors.
Women under the age of 35 have the highest success rates – another reason to think ahead about fertility. Unfortunately, the chances of success over the age of 40 are small.
How much does PGT cost?
PGT is expensive. The cost is somewhere in the region of US $20,000 (£15,000 or €18,000) for each attempt.
Health insurance usually doesn’t cover the cost of PGT/PGD. In some countries the public health care system will fund some PGT attempts – for instance, three attempts in the UK – but even this can vary within individual countries, and may be limited to couples with no existing children.
Any additional embryos that are not at risk of HD may be stored. However, this also comes with a cost that varies depending on the length of storage.
If this is an option you are considering, we recommend contacting your local genetic service to have a discussion regarding eligibility, referral, and associated costs.
Testing during Pregnancy
It is possible to perform a genetic test during pregnancy to see whether the developing baby (fetus) carries the gene expansion that causes HD. This is called prenatal testing.
Deciding whether to test a fetus is a difficult decision. It is important to understand that prenatal testing in HD is only performed on the understanding that if the result showed that the fetus carries the HD gene expansion, the pregnancy will be terminated. This is an immensely challenging and personal choice.
It’s important to think carefully about prenatal testing for HD, and how you feel about pregnancy termination, in advance of getting pregnant.
Once pregnant, there is very little time to absorb the information about the prenatal test and make these important decisions, as the testing has to be carried out early during a pregnancy.
Testing a pregnancy, but not going ahead with a termination after a positive test result, would take away the child’s right to choose whether to have the genetic test, later in life. After all, most people at risk of HD choose not to have the test before they develop symptoms. We know that big problems can occur when a child is identified, from birth, as someone who will develop HD.
In addition, most testing in pregnancy can only be done if tests have been carried out on the couple or other family members beforehand. Often, there is not enough time to do this background work when a pregnancy has already started.
Invasive Prenatal Testing
Most commonly and reliably, a procedure called chorionic villus sampling or CVS is performed during early pregnancy to test the fetus. CVS involves collecting a small sample of the placenta which represents the DNA in the fetus.
CVS is a quick procedure in the outpatient clinic, and in some countries, it is done under local anaesthetic. Depending on where the placenta is attached to the wall of the uterus, a very fine needle is passed either through the cervix or through the skin of the abdomen, using an ultrasound scanner to guide it. A small sample of cells is then collected from the placenta.
These cells can be used to test for the HD gene expansion. Some genetic centres will also offer testing for three common chromosome syndromes as part of the CVS genetic test.
CVS is usually carried out between 11 and 12 weeks into a pregnancy but no later than 15 weeks. An early dating scan is often required prior to a CVS taking place.
The main complication of this procedure is a small risk of miscarriage. Each centre will have specific information on the risk of miscarriage following a CVS. Please contact your local centre if you wish to learn more.
An amniocentesis is another type of invasive prenatal testing technique, similar to a CVS, but takes a sample of amniotic fluid rather than placenta. This can be carried out from 16 weeks. This therefore provides a result at a much later gestation and can make decisions around termination of pregnancy even more challenging.
If the genetic test is positive, a termination can usually be carried out under general anaesthetic until about 12-13 weeks depending on the country’s laws. Unfortunately, there can sometimes be a waiting list for this procedure.
In some countries termination of pregnancy may be carried out later on by inducing labour; however the availability of this option is again dependent on the country’s laws.
What if I don’t want to get the gene test myself?
There are ways to have HD-free kids without the at-risk partner getting tested themselves.
They use the same basic methods we’ve described – prenatal testing or PGT – with a genetic twist to identify ‘high risk’ pregnancies or embryos without revealing the HD gene status of the at-risk partner.
The twist is a couple of methods called exclusion testing or non-disclosure testing. These involve more preparation and planning, and there are some situations where it isn’t possible, so if this sounds like the right option for you: get expert advice early.
How does exclusion testing work?
Exclusion testing involves at least three blood samples. One each from the couple wanting to extend their family and ideally one each from both the mother and father of the person at-risk of developing HD. This technique may sometimes not be an option without a blood sample from the parent affected with HD.
We know that each of us will inherit one copy of the HD gene from each parent. The affected grandparent will have one normal copy of the HD gene and one expanded copy of the HD gene. We can label these genes ‘AA’. We do not know which one of these has been passed onto their adult child – and that person does not want to get tested to find out.
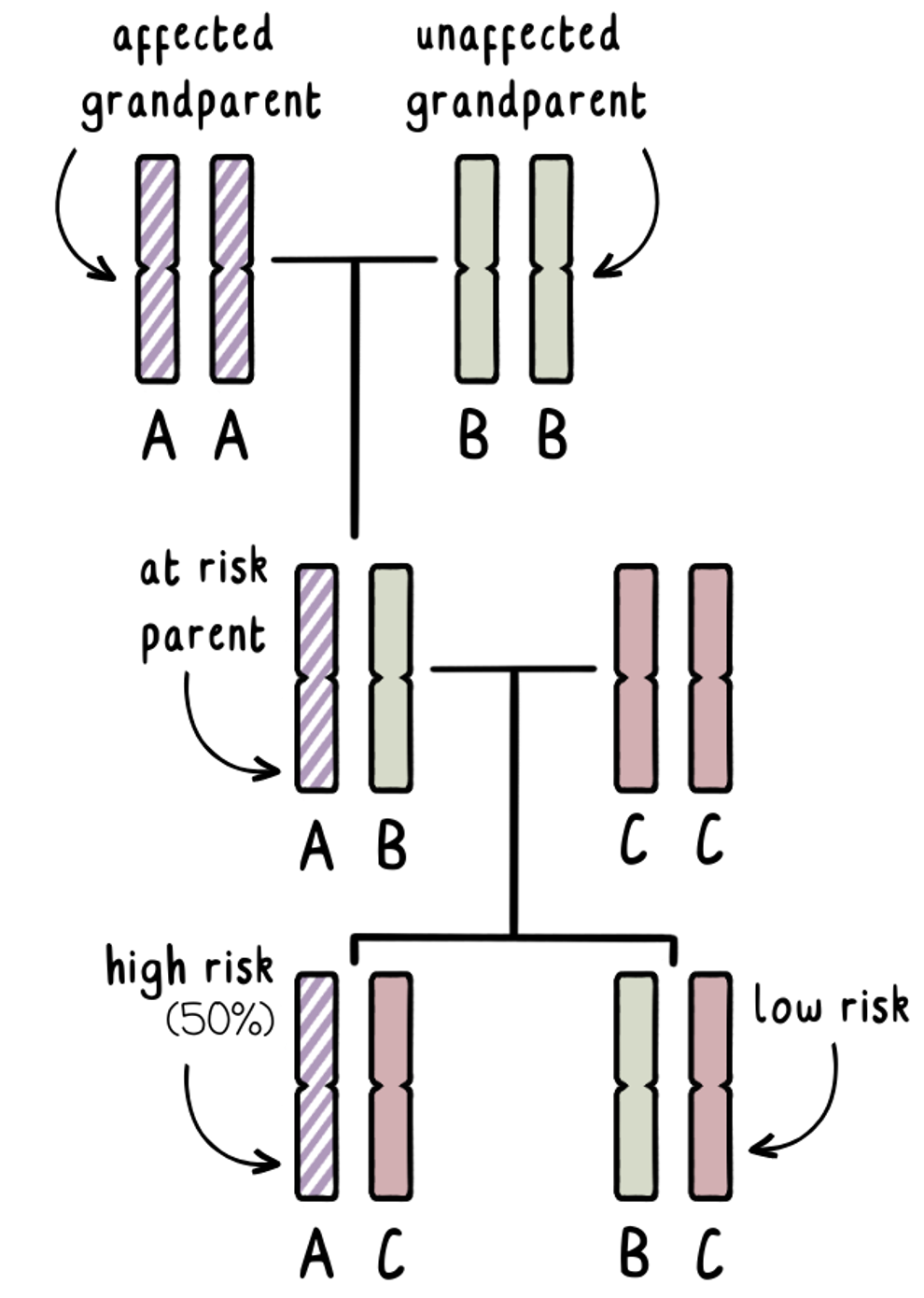
Image credit: @gcnotes
The unaffected grandparent will have two normal copies of the HD gene. We can call these ‘BB’.
The adult at risk will have some combination of A and B, with the A gene having a 50% chance of carrying the mutation.
If they wish to have a family without having genetic testing to determine their own risk, we can use exclusion testing during a prenatal test or PGT to identify if the fetus or embryo has inherited an A-gene from the affected grandparent, or a B-gene from the unaffected grandparent. This tells us whether the pregnancy would be high-risk or low-risk.
Crucially, exclusion testing identifies the grandparent of origin, without telling us whether the expanded HD gene has been inherited. If we found out the answer to that, it could tell us the results of the at-risk parent – which is what we are trying to avoid!
The flip side of this is that some high-risk embryos don’t carry an HD mutation, which would mean potentially ending a pregnancy or discarding embryos that may not have been at risk of HD in the first place.
Non-disclosure PGT
Non-disclosure is a twist on PGT that enables at-risk people to have HD-free children without finding out their own genetic status. This option is not available in every country, so it is important to contact your local genetic service to know if this is an option that is available in your area.
If an at-risk couple opt for non-disclosure PGT, the blood sample of the at-risk person would be tested for the HD mutation. The at-risk person would not be told the result of this test, and neither would any of the healthcare professionals that the at-risk person meets – only the professionals at the fertility lab would know the result.
The PGT then begins, with egg collection and generation of embryos. If the at-risk person’s ‘secret’ test result showed they had a HD gene expansion, the embryos are the tested for HD, and only those without the HD gene expansion are transferred for a potential pregnancy.
The couple isn’t told how many eggs are harvested, how many are successfully fertilised, or how many embryos are implanted. If there are no embryos without a HD gene expansion, the cycle stops there, and the couple are told that the fertilisation failed, but not why.
IVF can fail for many reasons, so a failure to get pregnant can’t be interpreted to mean the at-risk person has the HD gene.
Other options
One way to have HD-free kids is to use donor eggs or sperm instead of those of the at-risk person. Deciding to have a child with the help of a donor is a difficult decision but avoids the need to consider termination of a pregnancy. It can be done for people who’ve had a predictive test showing they carry an HD gene expansion, as well as those at risk who don’t want to be tested themselves.
Like all choices, this is complicated. The child won’t be genetically related to the at-risk parent, and careful thought will need to be given to how and when to share the information with the child. A parent doesn’t need to be genetically related to their child in order to fulfil a complete and loving parental role. There is plenty of support available to people who decide to go down this route, and this can be discussed before deciding to embark on the process.
Many couples think about adopting children. In many places, couples with one partner at risk of HD may have difficulty adopting a child. This is due to the disease being in the family and the adoption agency have to ensure the child has a stable home to go to. However, each case is individually assessed, so it is worth looking into adoption as an option. If you have been turned down for adoption, at-risk couples may be able to be foster carers for children as this is often a short-term option, caring for children over weeks or months at a time. The time you spend with foster children while short, can still often have a positive impact on the child’s life.
Future Directions
Non-invasive Prenatal Diagnosis is a newer way of testing in pregnancy without doing an invasive test, and so avoiding the small risk of miscarriage. Instead of an invasive test that takes a sample from the placenta or amniotic fluid, NIPD takes a blood sample from the parent carrying the pregnancy. This test looks for tiny bits of DNA from the fetus that float around in the parents’ blood.
NIPD can take place from around 10 weeks of pregnancy. NIPD usually involves some workup by the lab in advance of a pregnancy. It requires samples from the couple looking to extend their family and may require a sample from an affected relative.
NIPD is already available in the clinic for some inherited disorders and is in development for HD but not currently widely available. There are a number of instances where NIPD would not be appropriate, for example during twin pregnancies. If and when NIPD for HD is available, it is likely that a result indicating a pregnancy is at risk of HD may still be followed up with an invasive test to confirm the test results, before booking a termination.
What about LGBTQIA+ people?
All the options discussed above are likely to be available for LGBTQIA+ couples, with a family history of HD, that are looking to start a family. There would be the additional step of finding a sperm or egg donor as well as a surrogate, if necessary, which will come with its own additional cost and legal paperwork.
In many countries being LGBTQIA+ is unlikely to prevent you from accessing the family planning option that’s right for you and your partner. There will be specific information for the family planning techniques available in your country for LGBTQIA+ couples that wish to have a family.
Summary
There are a number of options available to people at risk of HD who wish to start a family.
Not everyone chooses to go through genetic testing to start a family, and this is a completely valid option.
For those who wish to remove the risk of their child inheriting HD, they may not need to know their own risk for HD. Direct testing can take place when we know the result of the at-risk parent and they are shown to have the HD gene expansion. Whereas exclusion or non-disclosure testing can be carried out for at-risk couples who do not wish to find out their own test results.
Direct and non-disclosure testing can take place during pre-implantation genetic testing (PGT) where embryos are created in the lab and tested for their risk of developing HD, or a fetus can be tested during pregnancy. Testing in pregnancy can be invasive via chorionic villus sampling (CVS) or non-invasive (NIPD), but both of these are only options for those who would consider ending a pregnancy at risk of developing HD.
There are other options available for at-risk couples that include using donor eggs/sperm or adoption/fostering of children.
Expert advice, in the form of genetic counselling, will help you understand the exact options available to you locally and help explore which option feels right for you. Your country’s HD Association can tell you how to get in touch with a genetic counsellor. As with so many things in life, forward planning and understanding all the options in advance is key.
Learn more
- Genetic Alliance (UK) page on preimplantation genetic testing
- National Society of Genetic Counselors (USA). Find a genetic counselor online. Your GP, family doctor or regional HD Association can also advise you on getting referred to a genetic counselor to discuss fertility options.
- The Human Fertility and Embryology Authority’s guide to assisted fertility for people living with genetic conditions (UK)
For more information about our disclosure policy see our FAQ…


